First published November 23, 2023 by DNDi.
The new results from Sudan, the country with the most documented mycetoma cases in the world, offer hope to patients who have suffered decades of neglect.

Results from the world’s first double-blind, randomized clinical trial to find a treatment for the fungal form of a chronic disabling disease called mycetoma have demonstrated that a new inexpensive oral treatment, fosravuconazole, is safe, patient-friendly, and effective in treating the disease.
The results of this Phase II clinical trial were announced by the non-profit medical research organization Drugs for Neglected Diseases initiative (DNDi) at the 13th European Congress on Tropical Medicine and International Health (ECTMIH) in Utrecht, Netherlands. DNDi coordinated the trial in Sudan in partnership with the Mycetoma Research Center (MRC) in Khartoum, Erasmus MC in the Netherlands, and the Japanese pharmaceutical company Eisai Co., Ltd. (Eisai).
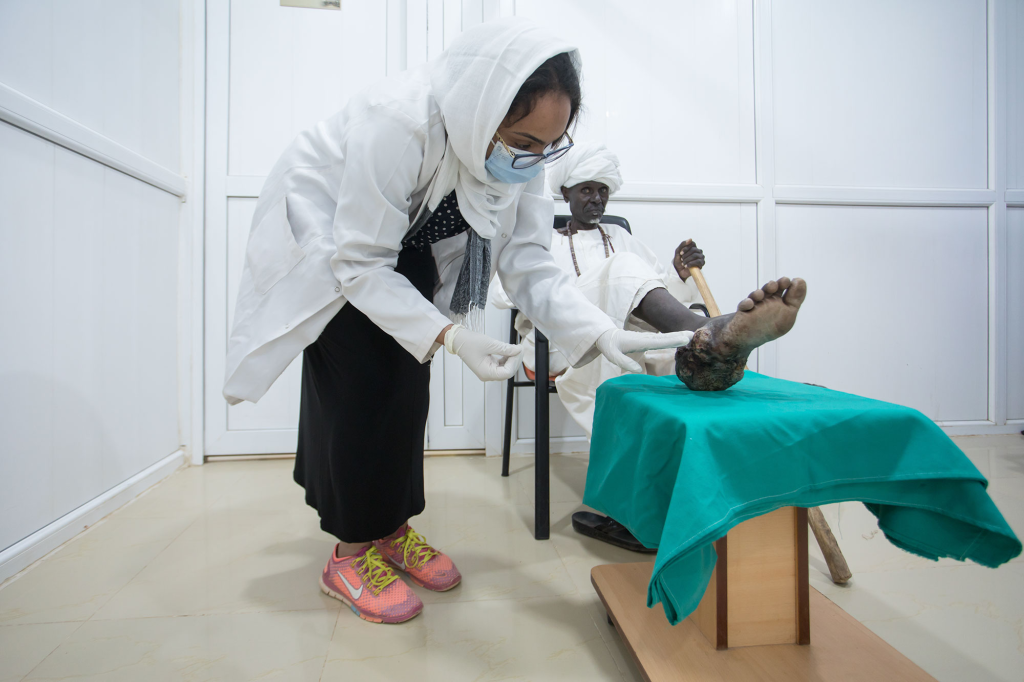
Mycetoma is a chronic infection caused by bacteria (actinomycetoma) or fungi (eumycetoma) and is one of the most neglected diseases in the world. It is endemic in many countries across Africa, Asia, and the Americas, with Sudan and Mexico reporting the highest number of cases worldwide.
The disease is contracted through implantation, meaning that people become infected through thorn pricks while walking barefoot. It primarily affects young adults, with children making up about 20-25% of all mycetoma patients, according to the MRC.
It usually (but not exclusively) affects the feet and leads to an invasive infection affecting the soft tissues and bones. Over time, severe deformities may occur, leading to considerable disability. Mycetoma can also lead to serious mental health issues due to the stigma associated with the chronic, non-relenting course of the disease and the absence of effective treatment, which often leads to amputation.

‘Mycetoma affects the poorest populations in the most remote areas,’ said Professor Ahmed Fahal, Professor of Surgery at the University of Khartoum and MRC Director. ‘Those who move around barefoot are the most at risk, mostly field labourers and children. Treating it can be a challenge due to the expensive and often unavailable treatments that also have serious side effects, and treatment lasts many months. The disease can cause grave complications and even death. In severe cases, the only solution is often amputation.’
In 2017, DNDi, in partnership with the MRC and Eisai, launched a clinical trial for fosravuconazole, a new antifungal treatment. Discovered by Eisai, the drug was later developed for patients with onychomycosis (fungal nail infection). However, the trial with MRC and Eisai was the first-ever double-blind, randomized clinical study for eumycetoma. It aimed to assess the superiority of fosravuconazole over itraconazole, which is the standard of care in endemic areas.
Results from the clinical trial showed that both drugs had similar efficacy rates. Fosravuconazole had an efficacy rate of 65% and 85% in the 300-milligram arm and the 200-milligram arm, respectively, while the itraconazole 400-milligram arm had an efficacy rate of 80%, which was higher than expected based on historical data. While the difference between these efficacy rates was not found to be statistically significant, fosravuconazole has significant advantages over the standard of care.
‘Only two pills of fosravuconazole need to be taken once a week, compared to four tablets per day for itraconazole. This reduces the pill burden for patients significantly, with only eight tablets per month versus the current 120 tablets per month. This will greatly improve adherence and convenience for patients,’ said Professor Fahal.

‘Besides the only once-a-week dosing, fosravuconazole offers several advantages over existing treatments,’ said Dr. Borna Nyaoke, Head of the Mycetoma Disease Programme at DNDi. ‘It has no food effect, so patients do not have to worry about taking it before or after a meal. The drug also has minimal interactions with other medications, which is particularly important for the sub-Saharan population that may suffer from comorbidities like HIV or tuberculosis and may be taking multiple drug combinations. All this makes fosravuconazole an attractive alternative treatment option for mycetoma.’
DNDi and its partners have presented the results of the clinical trial to the National Medicines and Poisons Board in Sudan. Due to the public health importance of this neglected disease, the Ministry of Health in Sudan has provided permission for use of fosravuconazole under controlled conditions to treat patients with mycetoma receiving care at the MRC. However, the ongoing conflict may hinder Sudanese patients from accessing the necessary treatment.
[Unfortunately, because of the war in Sudan, the MRC has had to shut down its clinic and research labs, according to the Royal Society of Tropical Medicine and Hygiene. “The catastrophic impact on patients’ care is multifaceted, ranging from the scarcity of essential medications to the disruption of treatment protocols. Patients, who were once beneficiaries of comprehensive and free management now face uncertainty and a lack of access to the vital care they desperately need.”
In addition, the MRC, “previously at the forefront of groundbreaking studies and innovative approaches to combat mycetoma, now finds its laboratories silenced and its research initiatives abruptly halted,” they wrote.]
‘Mycetoma is a unique and neglected tropical disease that disproportionately affects the most vulnerable populations. We have achieved a significant milestone by developing fosravuconazole, which will simplify fungal mycetoma treatment with its favourable pharmacokinetic properties,’ said Dr. Kappei Tsukahara, DHBL Microbes & Host Defense Domain Head and Head of Tsukuba Research Laboratories at Eisai. ‘We will work with our partners to make fosravuconazole available to all who need it, as soon as possible.’
The global burden of mycetoma is currently unknown due to a lack of surveillance and resources to conduct research on the disease. This has resulted in limited epidemiological data. However, DNDi and its partners aim to fill this knowledge gap by assessing the local needs for treatment in key endemic countries through literature review, retrospective data collection, and epidemiological field surveys.
Financial support for this study was provided by the Global Health Innovative Technology Fund (GHIT), Japan; the Swiss Agency for Development and Cooperation (SDC), Switzerland; the Republic and Canton of Geneva, International Solidarity Service, Switzerland; the Dutch Ministry of Foreign Affairs (DGIS), The Netherlands; UK aid, UK; Médecins Sans Frontières International (MSF); and MSF Switzerland.
Watch this video about mycetoma in Sudan:
